Published Date: Jan 2025
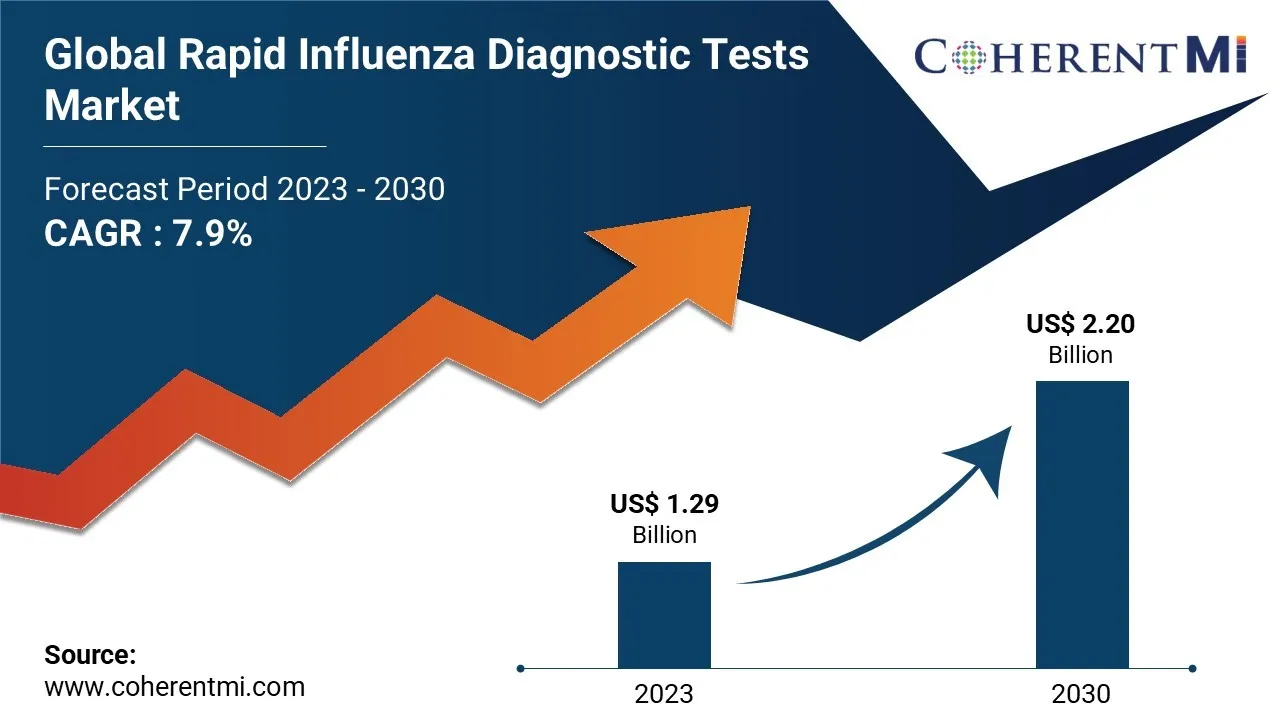
The rapid influenza diagnostic tests market size is estimated to be valued at US$1.29 Bn in 2023 and is expected to exhibit a CAGR of 7.9% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
Key Market Takeaways:
The global rapid influenza diagnostic tests market is anticipated to witness a CAGR of 7.9% during the forecast period 2023-2030, owing to the increasing prevalence of influenza infections and growing awareness regarding early diagnosis.
- On the basis of test type, rapid molecular assays segment is expected to hold a dominant position, accounting for the largest market share owing to benefits such as higher sensitivity and accuracy compared to lateral flow immunoassay tests.
- On the basis of influenza type, influenza type A segment is expected to hold a dominant position over the forecast period, due to its high transmission and infection rates.
- On the basis of specimen type, nasopharyngeal swab segment is expected to hold a dominant position, as nasopharyngeal swabs are considered as gold standard for influenza diagnostic sample collection.
- On the basis of end user, diagnostic laboratories segment is expected to be the leading segment owing to large number of test samples processed in diagnostic labs.
Rapid Influenza Diagnostic Tests Market Report Coverage
|
Report Coverage |
Details |
|
Market Revenue in 2023 |
USD 1.29 Billion |
|
Estimated Value by 2030 |
USD 2.20 Billion |
|
Growth Rate |
Poised to grow at a CAGR of 7.9% |
|
Historical Data |
2018–2022 |
|
Forecast Period |
2023–2030 |
|
Forecast Units |
Value (USD Million/Billion) |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Test Type, By Influenza Type, By Specimen Type, By End User |
|
Geographies Covered |
North America, Europe, Asia Pacific, and Rest of World |
|
Growth Drivers |
• Increasing Prevalence of Seasonal Influenza • Growing Demand for Point-of-Care Influenza Testing |
|
Restraints & Challenges |
• Pricing Pressure due to Intense Competition |
Market Dynamics:
The rapid influenza diagnostic tests market is witnessing high growth owing to rising incidences of influenza and increasing awareness regarding the early detection of influenza.
According to the WHO, influenza epidemics result in about 3 to 5 million cases of severe illness globally and about 290,000 to 650,000 respiratory deaths annually. Rapid influenza diagnosis helps in early treatment and prevents complications related to influenza infection.
Furthermore, the increasing adoption of point-of-care testing and decentralized rapid influenza diagnostic testing is also propelling the market growth. However, lack of diagnostic infrastructure in developing regions may hinder the market growth.
Market Trends:
- Multiplex testing facilitates simultaneous detection of influenza A, influenza B, and RSV from a single patient sample. This reduces diagnostic time, cost, and sample volume requirements. Major players are focusing on developing and launching multiplex rapid influenza diagnostic tests to gain a competitive edge in the rapid influenza diagnostic tests market.
- Key players are offering self-testing rapid influenza diagnostic kits to improve access and decentralize influenza testing.
Market Opportunities:
- The demand for point-of-care diagnostic tests is increasing rapidly owing to the need for quick tests that can be conducted near the patients without the need to send samples to laboratories. Rapid influenza diagnostic tests offer the benefit of providing results within 15-30 minutes directly at the patient care settings.
- With rising life expectancy and growing proportion of geriatric population worldwide, the risk of influenza outbreaks is also increasing. This highlights the need for rapid diagnosis of influenza among the elderly using point-of-care tests. The growing elderly demography will continue to drive the demand for rapid influenza diagnostic tests during the forecast period.
Competitor Insights
Key players in the rapid influenza diagnostic tests market include:
- Quidel Corporation
- Becton Dickinson
- Thermo Fisher Scientific
- Abbott Laboratories
- Hoffmann-La Roche
- DiaSorin
- Luminex Corporation
- Meridian Bioscience
- GenMark Diagnostics
- Sekisui Diagnostics
- Virax Biolabs
Recent Developments:
- On February 7, 2023, Virax Biolabs Group Limited announced the signing of a purchase order with Cosmos Health to launch and market COVID-19 and Influenza A+B Antigen Combo Rapid Detection Kits.
- On February 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the Lucira COVID-19 & Flu Home Test.
- On November 3, 2022, Virax Biolabs announced the launch of their RSV-Influenza-COVID Triple Virus Antigen Rapid Test Kit in markets accepting the CE mark, including the European Union. These test kits are designed for both at-home and point-of-care settings, providing results typically within 15 minutes.
Market Segmentation:
- By Test Type
-
- Rapid Molecular Assays
- Rapid Immunoassays
- Others
- By Influenza Type
-
- Influenza type A
- Influenza type B
- Influenza type C
- Others
- By Specimen Type
-
- Nasopharyngeal swab
- Nasal swab
- Throat swab
- Others
- By End User
-
- Diagnostic Laboratories
- Hospitals & Clinics
- Research Institutes
- Others
- By Region:
-
- North America:
- U.S.
- Canada
- Latin America:
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe:
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific:
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East:
- GCC Countries
- Israel
- Rest of Middle East
- Africa:
- South Africa
- North Africa
- Central Africa
- North America:
Related Reports :
